It’s that time of year again.

It’s the end of the year and I’ve got Homo erectus on the brain somethin fierce. Our precedent-erect first popped up in Africa around 1.9 million years ago, quickly spread throughout much of the Old World, and persisted until perhaps as late as ~ 100,000 years ago in Java, Indonesia. This was a very successful species by all accounts, and as a result of its great range and duration, you can imagine it was also pretty variable.

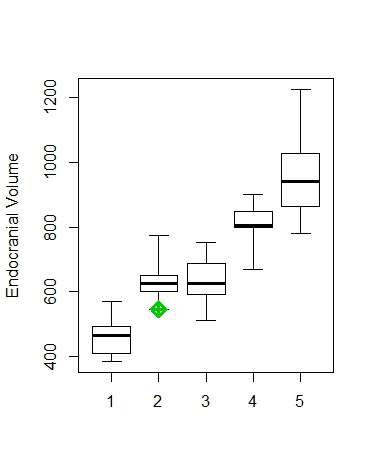
Hominin brain sizes. Boxes and whiskers represent sample tendencies and points are individual specimens. 1 = Australopithecus, 2 = Early Homo (cf. habilis & rudolfensis), 3 = Dmanisi H. erectus, 4 = Early African H. erectus, 5 = Early Indonesian H. erectus, 6 = Chinese H. erectus, 7 = Later Indonesian H. erectus, 8 = modern humans.
Despite this great variation, H. erectus skulls generally share a common visage: long and low cranial vault, low forehead, protruding brow ridges, fun tuberosities and tori in the back. You’d recognize them anywhere. Including the sidewalk!

Homo erectus in front of Ploenchit Tower, Bangkok (lateral view, front is to the right).
The relief in this sidewalk slat superficially looks like a trace fossil of partial H. erectus cranium, the face either missing (from the lower right) or taphonomically displaced toward the left side of the tile (see here for actual H. erectus trace fossils). This looks really similar to H. erectus from Indonesia, not surprising given its discovery in Thailand. Why, it could have come straight out of Figure 6 from a 2006 paper by Yousuke Kaifu and colleagues:

Left lateral views of Javanese H. erectus crania, modestly modified from Kaifu et al. (2006: Fig. 6). Front is to the left this time.
Using my insane photo editing skills, I’ve inserted the Ploenchit Tower trace fossil (reversed) within the horde of heads presented by Kaifu et al., above. Like many of the real fossils, the Ploenchit specimen is missing the face (due to taphonomy), the supraorbital torus or brow ridge juts out from a low-rising forehead, and the occipital bone also projects out about from the otherwise rounded contour of the cranium. Note that there is a good deal of variation in each of these features among the real fossils.
What a happy holiday accident to find a Homo erectus cranium on the street!

![]() Reference
Reference
Kaifu Y, Aziz F, Indriati E, Jacob T, Kurniawan I, & Baba H (2008). Cranial morphology of Javanese Homo erectus: new evidence for continuous evolution, specialization, and terminal extinction. Journal of human evolution, 55 (4), 551-80 PMID: 18635247