It will be lots of work to prep my Human Evolution course for the Fall. This past year has seen many major fossil discoveries, and adding to the list is the newly described species Australopithecus deyiremeda (Haile-Selassie et al., 2015). The fossils come from newly announced sites in Ethiopia (here it is on a map!), dating to around 3.4 million years ago. These new fossils are contemporaneous with Australopithecus afarensis, fossils attributed to Kenyanthropus platyops, and whatever the hell the Burtele foot belongs to.
The main specimens are a fairly complete half of a maxilla (upper jaw) and two decent mandibles (lower jaw bones). These fossils do not belong to the same individual (despite all the media pictures of the upper and lower jaws together). One of the most distinctive features of these fossils is how thick, both in absolute and relative terms, the mandibles are, especially given how short they are. What sticks out to me though, is that the upper jaw looks like it might have still had some growing to do. Why on earth would I think so? (The following is based off pictures from the publications, so I could be wrong!)

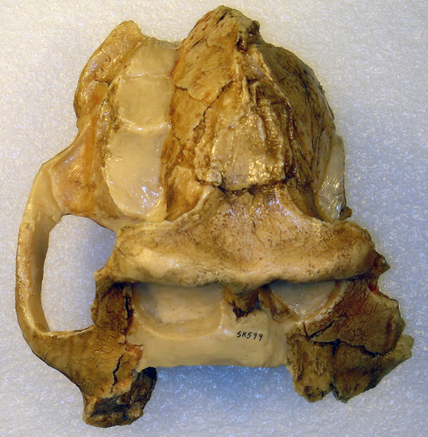
Extended Figure 1a from the paper, the type specimnen BRT-VP-3/1 maxilla viewed from the left side. I’ve added the M2 label for your reading pleasure.
The holotype maxilla (BRT-VP-3/1) is described as coming from a “young adult” in the Supplementary Information. However, it looks like the second molar tooth (M2) is not quite fully erupted and in occlusion, although this could be due to the natural arc of the tooth row. There is no visible wear on the tooth in the pictures, and indeed the Supplementary Information says the tooth is unworn. This means that the tooth is only recently emerged, and may not have passed the gum line, and therefore hasn’t seen much/any use yet. Authors note in the Supplementary Information that there is no M3 (a.k.a. “wisdom tooth”) wear facet on the back of M2 , meaning the last tooth hadn’t yet emerged yet either. So, this all points to a non-adult age by tooth eruption standards.

Extended Figure 1d from the paper. Same fossil, but from the bottom; pretend you’re a dentist peering into its mouth. Back is to the bottom.
In addition, the M2 roots don’t look fully formed. This is especially apparent in Extended Figure 1h, a CT section through the teeth:

Left side: Extended Figure 1h from the paper. From left to right, the teeth are P3, P4, M1, and M2. For comparison, to the right are Demirjian tooth development stages, modified from Table 2 of Kuykendall, 1996. Also compare the M2 roots with completed roots of the M1.
In many human populations, this stage of M2 development is reached (on average) between 11-13 years (Liversidge et al., 2006). In the wild Taï Forest chimpanzee sample, two individuals with M2 root completely formed (Stage H) are 10 and 11 years old (Smith et al., 2010). These apes would not be fully mature and their facial dimensions would likely have increased had they reached adulthood (Zihlman et al., 2007).
So what this suggests to me is that this maxilla may not accurately represent adult anatomy in this newly described species. In humans, the face continues to grow downwards from adolescence into adulthood, and in apes the face continues growing both forward and downward. In the differential diagnosis of A. deyiremeda, Haile-Selassie and team state, in layman’s terms, that the cheeks are positioned more toward the front than in A. afarensis, and that the front of the face doesn’t stick out as much as in A. garhi. If this specimen was not fully grown, it is likely that the true adult anatomy would have had a face that sticks out more and has less forward-positioned cheeks than in this specimen.
But, this is all speculative, and I’d like to reiterate that these observations of dental development are based only on the published pictures. Just a thought!